Cranial remolding expert Ellie Boomer, CPO, shares her commentary on the helmet therapy article highlighted in the May 1 issue of The New York Times.

As a professional who specializes in
treating children with cranial deformation
by using a cranial remolding
orthosis (CRO), I was surprised to see The New York Times (NYT)
article “Helmets Do Little to Help Moderate Infant Skull Flattening,
Study Finds,” which discussed a study from the Netherlands
that called into question the efficacy of CROs. For a moment, I
was forced to consider the possiblity that I have been correcting
cranial deformations that would have corrected on their own.
Over the last 12 years, I have seen effective correction of
cranial deformation when parents and caregivers are compliant
with a 23-hours-per-day, full-time wear schedule with a
well-fitting, active-design CRO. I have also remeasured infants’
skulls after they were involved in repositioning programs and
then recommended CRO treatment because the deformational
asymmetries failed to correct with that protocol. Also, in
follow-up with patients one to two years post-CRO treatment,
I have typically seen the head increase in size, a slight decrease
in the cephalic ratio/cranial index, and the cranial vault holding.
Thus, even though Mark Proctor, MD, vice chair of neurosurgery
at Boston Children’s Hospital, Massachusetts, and an assistant
professor at Harvard Medical School, Boston, was quoted as
saying that the study was “rigorous,” my own clinical experience
led me to believe that there were factors in this study that had
not been included in the NYT article’s summary of methods and
results that contradict Proctor’s assessment.
The study referred to in the NYT article, “Helmet Therapy in
Infants with Positional Skull Deformation: Randomised Controlled
Trial,” by Renske van Wijk et al., was published online
May 1 in BMJ, an open-access, peer-reviewed journal. This is
the first randomized controlled trial on helmet therapy, and I agree with Proctor that the method of enrolling patients,
follow-up
with the enrolled patients, and methods of measuring
the asymmetries were strong.

However, the study’s conclusion that there “was no conclusive
evidence that a significant or clinically meaningful difference
in improvement of skull shape was found at the 24-month
follow-up between the two groups” is not consistent with my
own clinical results or with previous literature. It appears
that the reason van Wijk et al.’s study was unable to show the
effectiveness of treatment with a CRO lies in the many weaknesses
of the study, among which are the low participation rate,
incomplete reporting, dissimilar cohort groups, and questionable
averaging of data.
Study Introduction
The study cohort was found by asking parents of five-month-old
infants to participate in a randomized controlled study. Of the
initial 883 infants enrolled for baseline measurements, only 401
met the inclusion criteria, and only 84 parents (21 percent of the
eligible infants) agreed to participate in the study. This number
is low but perhaps understandable since the study parameters
included the possiblity the child would not receive treatment.
The 84 infants were randomly assigned (1:1) to either helmet
therapy for six months or no treatment, and were remeasured at
eight, 12, and 24 months of age. Primary outcomes were evaluated
using anthropometric measurements
at 24 months of age, which were
blinded at 24 months only. The measurements
were taken using plagiocephalometry
(PCM)-a thermoplastic material
that is wrapped around the child’s head,
at which time the position of the child’s
nose and ears are marked on the material.
This material is then photocopied and the
measurements are taken from the paper.
Previous scholarly literature has documented
the intrarater and interrater reliability
of this method.1
With the exception of the diagonal
measurements having been taken at 40
degrees from midline, instead of the
standard 30 degrees used in the United
States, and the plagiocephaly index having been calcuated differently
from the U.S. method of calcuating the cranial vault
asymmetry index (CVAI), the results provide similar measures
of plagiocephaly and brachycephaly, as shown in Table 1.
Table 1
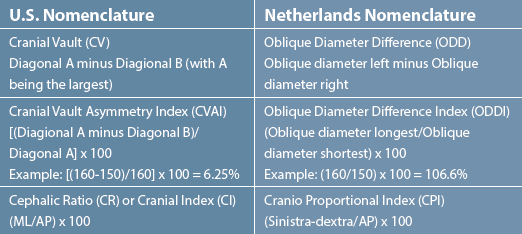
According to the study, the researchers had a 94 percent
follow-up rate, and they did statistical analysis on two outcome
variables: change in the oblique diameter difference index
(ODDI), which is equivalent to the U.S. definition of CVAI, at
24 months of age versus five months of age; and change in the
cranio proportional index (CPI), equivalent to the U.S. definition
of cephalic ratio (CR) or cranial index (CI), at 24 months of age
versus five months of age. The effectiveness of treatment was then
presented as the difference of these two variables in the helmet
treatment group versus the natural course group.
The study authors hypothesized that although helmet therapy
is expected to give slightly better results in the short term,
the natural course of skull deformation would catch up with the
effects of helmet therapy over time and that no clinically meaningful
differences would be present between the two groups at
two years of age.
Commentary on the Study Analysis
Of the children who were assigned to the helmet treatment,
it appears that there were inconsistencies with regard to the
brand of helmet used, who delivered the treatment, and the facility
at which the patients were treated.
Because the study mentions two brands
of custom-made helmets that use the
“same mechanism to redirect skull
growth” but does not list the brands
or fabrication methods, it is not reproducible.
Further, some of the ill-fitting
helmets depicted in a press release for
the study represent a passive-design
CRO, use chin straps, and appear to
be excessively large. This type of design
is not comparable to the active-design
CROs that are typically used in the
United States (represented in the photos
that accompany this article), which
have neither a chin strap nor move
excessively. The study does not contain
details of the material used to fabricate
the CROs; impression methods (cast,
scan, or measurements); modification
methods (reliefs and/or build-ups on the molds and
modifications to the helmet throughout treatment); or
how the modifications differed between patients with
plagiocephaly and brachycephaly. Nor is there commentary
about how many orthotists were involved, or
their training or CRO experience. Additionally, the
patients treated were seen in four different institutions.

The study notes that all patients assigned to helmet
therapy had one or more side effects-an outcome
contrary to existing literature, which puts the incidence of side effects closer to 25 percent.2 The study attributes
this difference to the researchers’ broader definition of
side effects, including sweating and unpleasant odor; however,
only 23 percent were related to skin irritation while 73 percent
of the side effects were associated with fit problems. One parent
even reported that the helmet came off spontaneously. I have
not heard of any experienced orthotists who encounter CRO
fit issues in three out of every four of their CRO patients. This
indicates poor-quality helmets rather than an increase in side
effects, and further weakens the study’s conclusions. As a result
of side effects reported in the study, ten parents discontinued
helmeting before their infants reached 12 months of age. However,
all infants assigned to the CRO group were included in
the calculations.
The study’s reporting on the number of
children that actually received treatment
is unclear. It states that of the 42 children
initially assigned to helmet therapy, six
infants did not start treatment. (In three
cases the parents preferred no treatment,
and in three other cases the physician
advised against helmet therapy.) Further,
there was an infant who was allocated to
the natural course whose parents preferred
helmet therapy. But again, it is unclear if
this child’s results were included in the
treated or untreated group. This means
we would expect that either 36 or 37
infants were fitted with a CRO. What is
further confusing is that the study states
that only “ten of 30 infants received helmet
therapy until 12 months of
age.” So is the number of infants
who actually underwent treatment
only 30? And if so, what
happened to those other six or
seven children? Basically, the
reported numbers for treated
infants do not add up.
It is also difficult to prove
or disprove the first part of the researchers’ hypothesis-that
they expected to see slightly better results at the eight- and
12-month follow-ups compared to the 24-month follow-up
since they failed to report their short-term follow-up results.
In addition, the helmet treatment was described as starting in
infants no later than six-and-a-half months of age, and families
were instructed to have their infant wear the helmet for 23 hours
per day until 12 months of age. At six months of age, a moderate
asymmetry does not usually indicate full-time treatment for
six months. In my experience, at six months of age, treatment of
a moderate asymmetry should take about three to four months
with full-time wear of an active-design CRO, and compliance
is critical to achieve these results. The study protocol included
monitoring compliance using an electronic device built into
the helmet; however, the study authors stated that “data from
the measuring devices proved to be unreliable and we therefore
omitted them from further analysis.”
Although the researchers relied on a parent questionnaire
to measure compliance in absence of the measuring devices,
a skilled orthotist can tell from hand measurements and the fit
of the orthosis if the family has been compliant with full-time
wear. However, the study fails to provide specific information
regarding follow-up care with the orthotist. It does mention
that the “helmet was modified or replaced to accommodate
skull growth,” but no details are provided, nor does the study
account for how many helmets were replaced.
Even if we were to ignore all of the obvious CRO fit and followup
issues and assume a perfectly designed and fitted orthosis,
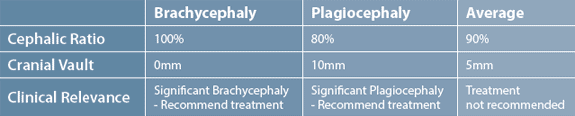
any clinical relevance to the study
is removed because the final statistical
analysis involved averaging
the results for the three different
conditions (plagiocephaly, brachycephaly,
and combination). Table
2 demonstrates how averaging
different head-shape measures
dilutes the results and therefore
the clinical relevance. To have
a clinically relevant study, the
researchers should have changed
their inclusion criteria to only
one head-shape type-or they
should have had a larger patient
population to show statistical
significance with each of the three
head-shape types.
Table 2
Support authors and subscribe to content
This is premium stuff. Subscribe to read the entire article.




