For the first time, researchers recorded pain-related data from inside the brain of an individual with post-amputation phantom limb pain. A long sought-after goal has been to understand how pain is represented by brain activity and how to modulate that activity to relieve suffering from chronic pain.
The researchers collected data from the patient with phantom limb pain and three patients with post-stroke pain. Data collection was done from the participants’ homes and analyzed using machine learning tools to identify an area of the brain associated with chronic pain and objective biomarkers.
The findings, which represent a first step towards developing novel methods for tracking and treating chronic pain, were funded by the National Institutes of Health (NIH) Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative and the Helping to End Addiction Long-term Initiative (HEAL Initiative).
“This is a great example of how tools for measuring brain activity originating from the BRAIN Initiative have been applied to the significant public health problem of relieving persistent, severe chronic pain,” said Walter Koroshetz, MD, director of the National Institute of Neurological Disorders and Stroke, part of NIH. “We are hopeful that building from these preliminary findings could lead to effective, non-addictive pain treatments.”
Neuropathic pain most commonly occurs due to injury to the nerves in our bodies, but for the individuals in the study, their pain is thought to originate from the brain itself, which doesn’t respond well to current treatments.
“When you think about it, pain is one of the most fundamental experiences an organism can have,” said Prasad Shirvalkar, MD, PhD, associate professor of anesthesia and neurological surgery at the University of California, San Francisco, and lead author of the study. “Despite this, there is still so much we don’t understand about how pain works. By developing better tools to study and potentially affect pain responses in the brain, we hope to provide options to people living with chronic pain conditions.”
Traditionally, researchers gather data about chronic pain through self-reports from those living with the condition. This study also looked directly at changes in brain activity in two regions where pain responses are thought to occur—the anterior cingulate cortex (ACC) and the orbitofrontal cortex (OFC)—as participants reported their current levels of chronic pain.
“Functional MRI studies show that the ACC and OFC regions of the brain light up during acute pain experiments. We were interested to see whether these regions also played a role in how the brain processes chronic pain,” said Shirvalkar. “We were most interested in questions like how pain changes over time, and what brain signals might correspond to or predict high levels of chronic pain?”
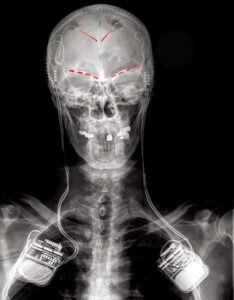
The participants were surgically implanted with electrodes targeting their ACC and OFC. Several times a day, each participant was asked to answer questions related to how they would rate the pain they were experiencing, including strength, type of pain, and how their level of pain was making them feel emotionally. They would then initiate a brain recording by clicking a remote-control device, which provided a snapshot of the activity in the ACC and OFC at that exact moment. Using machine learning analyses, the research team was able to use activity in the OFC to predict the participants’ chronic pain state.
“The device we implanted into the participants in this study is actually a deep brain stimulation device that has the capability not only to sense and record brain activity, but also to provide electrical stimulation when needed,” said Philip Starr, MD, PhD, professor of neurological surgery and a senior author of the study. “The hope is that by pinpointing the specific brain signals underlying someone’s pain experience, we can program the device to deliver stimulation only when those signals are detected and return the brain’s pain networks to a normal, healthy state.”
In a separate study, the researchers looked at how the ACC and OFC responded to acute pain, which was caused by applying heat to areas of the participants’ bodies. In two of the four patients, brain activity could again predict pain responses, but in this case the ACC appeared to be the region most involved. The research teams say this suggests that the brain processes acute and chronic pain differently, though more studies are needed given that data from only two participants were used in this comparison.
Identifying a pain signature will enable the development of new therapies that can alter brain activity to relieve suffering due to chronic pain. The most immediate benefit may be in informing ongoing studies in HEAL and BRAIN to employ deep brain stimulation to treat chronic pain. Ongoing and future work involving more participants will be key in determining whether different pain conditions share the OFC activity seen in these patients or how the signatures differ among persons with different pain conditions.
Effective and non-addictive treatments for chronic pain conditions is a main goal of NIH HEAL Initiative efforts to find scientific solutions to stem the opioid public health crisis. The findings are a key step to identifying pain-specific biomarkers toward personalizing pain management for individuals, leading to the development of new technologies and advances to better understand brain circuit.
Editor’s note: This story was adapted from materials provided by the National Institutes of Health and the University of California, San Francisco.
The study, “Brain Signatures for Chronic Pain identified in a Small Group,” was published in Nature Neuroscience.




