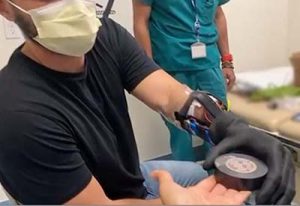
An interdisciplinary team at the University of California Davis (UC Davis) is collaborating to utilize targeted muscle reinnervation (TMR) to help people with upper-limb amputations better control their residual muscles, receive sensory feedback, and reduce limb pain. The team includes plastic and reconstructive surgeons, mechanical engineers, neuroscientists, orthotists, and prosthetists.
TMR transfers residual nerves from an amputated limb into a new muscle, allowing patients to move their prosthetic arm by thinking what movements they wish to accomplish.
“This endeavor requires a multidisciplinary approach,” said Clifford T. Pereira, MD, associate professor of plastic and reconstructive surgery at UC Davis Health. “By reassigning these existing nerves, we’ve been able to make it possible for patients to retain their nerve functions from the amputated limb that otherwise would have lost their function,” Pereira said. “
TMR can be used to treat pain from phantom limb and neuromas by creating a pathway for the nerves in the reinnervated muscle, which makes it less likely that they will develop painful scar tissue, a common problem for patients who have had amputations.
“It has become increasingly apparent that restoring the muscles to their normal physiology has benefits not only for prosthetic control, but also for a patient’s day-to-day mental well-being,” said Andrew I. Li, MD, assistant professor of plastic and reconstructive surgery, who performed the first TMR procedures with Pereira at UC Davis Health.
Wilsaan M. Joiner, PhD, associate professor of neurobiology, physiology and behavior and neurology, and Jonathon Schofield, PhD, assistant professor of mechanical and aerospace engineering, are also part of the project. They are developing a process using ultrasound and electromyography to detect the reconnected nerve signals based on muscle contractions. The data is then interpreted by artificial intelligence software, which decodes the intended motions of the hands and fingers.
“We can see different patterns in their muscle activity using ultrasound or electromyography and then use machine learning to predict what they’re trying to do with their missing limb,” said Joiner.
The technique offers advantages over surface electrodes, which can also pick up electrical charges from other muscles.
“The machine is interpreting the brain’s activity by monitoring the reinnervated (or rewired) muscles and showing us whether the patient wants to close their hand, point a finger, pinch an object, rotate the wrist, or bend their elbow,” said Schofield. “What’s exciting about this is the prosthesis will adapt to individual patients, rather than the patients adapting to the prosthesis as they have to now.”
Laduan Smedley, CPO, part of UC Davis Health’s department of physical medicine and rehabilitation, has begun working with patients who have tested Joiner and Schofield’s interface and has already seen better outcomes.
“The interface is a lot more intuitive, where a patient can just think about opening and closing their hand, as opposed to trying to correlate another movement to operate their hands,” said Smedley. “It may really improve the experience for amputee patients without having to memorize multiple control sequences.”
Editor’s note: This story was adapted from materials provided by UC Davis Health.




