When John German’s left arm was amputated below the elbow in 1987, he initially resisted the idea that he could no longer be left-handed. German, who had undergone 17 surgeries-one of which almost cost him his life-suffered from thoracic outlet syndrome, a congenital defect involving an extra cervical rib near his clavicle that was putting pressure on his left subclavian artery. Glad to be alive, he immediately enrolled in an in-patient rehab program but was kicked out for trying to make his prosthesis operate as his dominant hand. He subsequently taught himself to perform everyday tasks.
 |
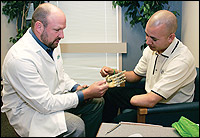
| John German, a long-time user of myoelectric-controlled prosthetic devices, has always been interested in the newest prosthetic technology. |
Today, the 40-year-old medical device salesman and clinical technician from Altoona, Pennsylvania, describes himself as someone who has adapted very well and jokes that he spent the first 20 years of his life left-handed and the second 20 years right-handed.
“I’ve done more with one hand than I ever dreamed I could have done with two,” he says.
Over the last 20 years, German has used a variety of hand solutions-from a passive silicone hand to the latest myoelectric-controlled hand. He liked the aesthetics of the silicone hand but wore it only for a short time because he needed something more functional to suit his active lifestyle. With a myoelectric-controlled hand, German says, “I’ve never really considered myself as very limited.”
Two weeks ago, German’s outlook shifted when he became part of a market preference study for Touch Bionics, Livingston, United Kingdom, to test its i-LIMB™ hand. The i-LIMB looks and acts like a real human hand, and is the first widely available prosthetic device with five individually powered digits. The product is being released in tandem with the ProDigits™ system, a powered partial-hand prosthesis.
“The mechanics of upper-extremity prosthetic components are finally beginning to approach that of our own hands and fingers,” says upper-extremity specialist Randall Alley, BSc, CP, LP, FAAOP, CFT, CEO of biodesigns Inc., Thousand Oaks, California. “Both the i-LIMB and ProDigit systems…provide a modular capability not fully realized with current technology. It is much more in sync with a clinician’s desire to provide custom solutions to a particular problem by allowing the patient’s needs to determine the final design as opposed to the limitations of current designs determining what needs can be fulfilled.”
The Story Behind the Technology
 |
| John German, who loves to cook, peels a banana with his i-LIMB hand. |
The roots of this technology-and Touch Bionics-can be traced back to 1963, when the National Health Service (NHS) in the United Kingdom launched a comprehensive research program to develop prosthetic solutions for children who had been affected by thalidomide. According to the U.S. Food and Drug Administration (FDA), thalidomide was first marketed in Europe in the late 1950s as a sleeping pill and to treat morning sickness in pregnant women. When pregnant women used thalidomide, it resulted in the birth of thousands of babies with deformities and other defects including abnormally short limbs, eye and ear defects, and internal defects of the heart, genitals, kidneys, and digestive tract.
“There were more than 20 kids born in Scotland with greatly impaired limbs,” says Stuart Mead, CEO of Touch Bionics. “The government decided to put aside special funds to develop prosthetics for these children as they grew up. One of the technologies that came out of that, in the late 1980s and early 1990s, was the idea of producing individually powered digits.”
Because the mission of NHS is to care for patients and not to develop and manufacture products, Touch Bionics was spun out from NHS in 2003. “One of the primary reasons for the spinout was to develop the technology and make it available to other patients-in our case, worldwide,” says Mead.
Collaboration Crosses Institutional and Geographic Lines
 |
| Juan Arredondo, a 27-year-old sergeant in the U.S. Army, now retired, is fitted with the i-LIMB hand. |
Bringing the technology to market has been a collaborative effort between corporate, commercial, academic, and independent entities, crossing not only these lines but also international lines. The initial research for the i-LIMB was conducted by David Gow, PhD, the director of rehabilitation engineering services at NHS Lothian, Scotland, and director of technology at Touch Bionics. Once Touch Bionics was formed, much of the early development work took place in consultation with Bill Dykes, SRPros MBAPO, a senior lecturer at the National Centre for Prosthetics and Orthotics at the University of Strathclyde, Glasgow, Scotland, which has one of the biggest prosthetic centers in the United Kingdom. “We utilized some of their expertise in order to get the product to a point where we could then introduce it to some of our partners in the U.S.,” says Mead.
Knowing that a successful commercial launch would require introduction into the U.S. market, Touch Bionics partnered with several U.S.-based clinical companies including Advanced Arm Dynamics, headquartered in Redondo Beach, California; Benchmark Medical, Malvern, Pennsylvania; Hanger Orthopedic Group Inc., Bethesda, Maryland; Prosthetic & Orthotic Associates International, Middletown, New York; and Scott Sabolich Prosthetics and Research, Oklahoma City, Oklahoma. The company also partnered with LIVINGSKIN, Middletown, New York, and ARTech Laboratories, Midlothian, Texas, to develop durable, lifelike cosmesis solutions.
Patient-Centered R&D
From the beginning, both Mead and Phil Newman, the head of sales and marketing at Touch Bionics, said that one of the company’s main priorities was to keep the product clinically relevant. “One of the key things we wanted to do is understand how the hand performs on patients,” says Newman.
“It’s very easy, if you’re not careful, to end up doing robotics rather than prosthetics,” Mead adds, “so we were really keen, all the way through the development process, to involve patients.”
Touch Bionics selected 12 persons-a mix of civilian, military, and ex-military patients from the United Kingdom and the United States-to participate in a market preference study. All of the patients are transradial amputees and current myoelectric users. “They were specifically selected as transradial because&we wanted to have a group where we could just assess the hand performance without being confused by any other prosthetic device,” says Mead.
When asked what he has been able to do with his new hand that he hadn’t been able to previously, German says, “It’s what the older technology has forced me to do in the way of adapting. For example, bending at the knees and shoulder to pick something up where a normal person would only have to bend at the elbow and wrist… Because the grip can match to the task that you’re doing, all that creative adapting isn’t necessary anymore.”
The Fourth Paradigm Shift
 |
| An assembled ProDigit. |
“From a patient perspective, this technology is breakthrough in terms of shifting the paradigm of how upper-extremity amputees can utilize a prosthesis,” says Tom Passero, CP, clinical director at Prosthetic & Orthotic Associates International Inc. and LIVINGSKIN. Passero, who has been involved with both the patient study and the cosmesis development for the i-LIMB hand and ProDigits system, detailed three paradigm shifts that he believes have changed the way amputees could engage the world while using a prosthesis: the development of the Flex-Foot®, now manufactured by Ossur, in the late 1970s; the development of silicone as an interface material in the early 1980s; and the inclusion of microprocessors in lower-extremity prosthetics, specifically the Otto Bock C-Leg®, in the late 1990s.
Passero says that the complex grasping pattern of the i-LIMB hand presents a fourth paradigm shift. “Aside from some subtle electronic changes, the grasping pattern, which is the all-important part of an external-powered hand, has been fairly simple,” he says. “With palmar prehension, there’s no articulation at the knuckles, no independent movement, no complex movement of the thumb-and that’s been the state of the art for 40 years. So this hand breaks that barrier, in my opinion.”
Troy Farnsworth, BSME, CP, FAAOP, director of Hanger Prosthetics & Orthotics Inc.’s National Upper-Extremity Prosthetic Program, agrees. “I think this new hand is going to force us to change the way we think about fitting electronic hand prostheses on patients, and the way manufacturers look at designing them.”
i-LIMB Grip PatternsThe i-LIMB has articulating fingers to close tightly around objects. Built-in stall detection tells each individual finger when it has sufficient grip on an object. Individual fingers lock into position until the user triggers a muscle to open the finger. |




