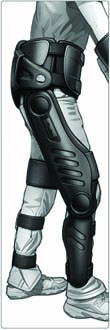
Sketch of the Parker-Hannifin design concept for the commercial version of the Vanderbilt exoskeleton, courtesy of Parker-Hannifin.
Vanderbilt University, Nashville, Tennessee, has announced that it has signed an exclusive licensing agreement with Parker Hannifin, headquartered in Cleveland, Ohio, for the company to develop a commercial version of the Vanderbilt exoskeleton. The agreement gives Parker exclusive rights to develop, manufacture, and sell the device. Parker said it intends to invest in further development of the technology and establish a business unit targeting commercial launch of the exoskeleton device in 2014.
The device was developed by Michael Goldfarb, PhD, the H. Fort Flowers Chair in Mechanical Engineering and professor of mechanical engineering and of physical medicine and rehabilitation at Vanderbilt, with the assistance of Vanderbilt research engineer Don Truex and Vanderbilt graduate students Hugo Quintero, Spencer Murray, and Kevin Ha, and Ryan Farris, a former student who now works for Parker. Funding for the project was received from the National Institutes of Health.
The Vanderbilt exoskeleton weighs about 27 pounds, nearly half the weight of the other exoskeletons. The other models are also bulkier so most users wearing them cannot fit into a standard-sized wheelchair, according to the Vanderbilt press release. A proprietary control interface allows for smooth operation that works in harmony with natural human movement and body position. It is also the only exoskeleton that incorporates functional stimulation. There is also the matter of cost. The price tags of other rehabilitation model exoskeletons have been reported to be as high as $140,000 apiece, plus an annual service fee. Parker Hannifin hasn’t set a price for the Vanderbilt exoskeleton, but Goldfarb said he is hopeful that its minimalist design combined with Parker Hannifin’s manufacturing capability will translate into a more affordable product. “It would be wonderful if we could get the price down to a level where individuals could afford them and insurance companies would cover them,” he said.
“This agreement offers Parker an exciting growth opportunity in the area of biomechanics,” said Craig Maxwell, vice president of technology and innovation for Parker. “By leveraging our core motion and control technology, we are confident that we can take the company in new and exciting directions while improving the lives of people who experience mobility challenges.”
The exoskeleton is currently being tested and refined through clinical research at the Shepherd Center, Atlanta, Georgia, a rehabilitation hospital that specializes in medical treatment, research, and rehabilitation for people with spinal cord injury or brain injury.




