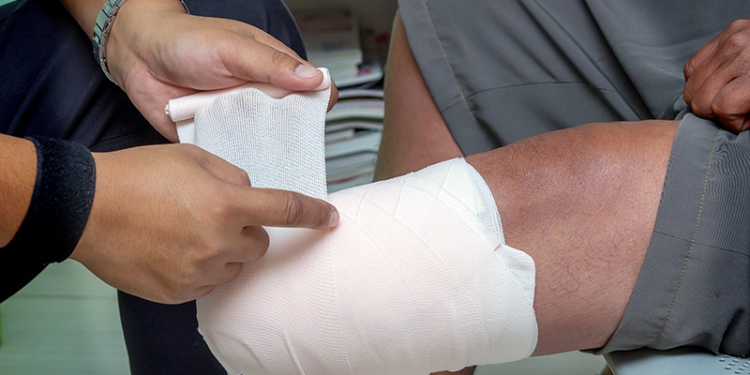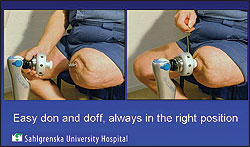
The U.S. Food and Drug Administration (FDA) has authorized use of the first bone-anchored prosthesis marketed in the United States for adults who have transfemoral amputations and who have rehabilitation problems with, or cannot use, a conventional prosthetic socket. For example, some patients may not have a long enough residual limb to properly fit a socket prosthesis or may have other conditions, such as scarring, pain, recurrent skin infections, or fluctuations in the shape of the residual limb that prevent them from being able to use a prosthesis with a socket. The Osseoanchored Prostheses for the Rehabilitation of Amputees (OPRA) device, which is manufactured by Integrum, Molndal, Sweden, uses fixtures and screws implanted into the patient’s remaining thigh bone to connect an external prosthetic limb.
The OPRA device is installed with two surgical procedures. In the first procedure, a cylinder-shaped fixture is implanted into the central canal of the remaining thigh bone. About six months later, after tissue has grown to anchor the fixture and the skin tissue has healed, a second surgery is performed to implant a rod that attaches to the fixture from the previous surgery. This rod extends through the skin at the bottom of the patient’s residual limb and connects to the prosthetic leg. After the second surgery, the patient works with a trained physical therapist to gradually place weight on the OPRA device using a training prosthesis. Patients require about six months of training and rehabilitation after the second surgery, before being fitted with their own customized prosthesis by a trained prosthetist.
The OPRA device received a Humanitarian Use Device (HUD) designation and was reviewed through the Humanitarian Device Exemption (HDE) pathway. An HDE is an application that is similar to a premarket approval application (PMA) but it is exempt from the effectiveness requirements that apply to PMAs. Devices are eligible for HUD designation if they are designed to treat or diagnose a disease or condition that affects or is manifested in fewer than 4,000 individuals in the United States per year. In order to receive HDE approval for a HUD, a company must demonstrate the safety and probable benefit of the device (i.e., that the device will not expose patients to an unreasonable or significant risk of illness or injury, and that the probable benefit of the device outweighs the risk of injury or illness from its use), and that there are no legally marketed comparable devices, other than a device approved under the HDE or investigational device exemption (IDE), available to treat or diagnose the disease or condition.
Data supporting the safety and probable benefit of the OPRA device included mechanical testing of the device’s parts when subjected to weight, twisting, bending, and simulated repeated use, and a two-year, 51-subject clinical trial. The clinical trial found that study subjects reported increased prosthetic use, and improved mobility, comfort, function, and quality of life compared to the subjects’ own outcomes prior to the surgeries. The most common adverse event was infection.