
The brain-spine interface is seen here with a microelectrode array and a silicon model of a primate’s brain, as well as a pulse generator used to stimulate electrodes implanted on the spinal cord. Photograph by Alain Herzog, Courtesy of EPFL.
An international team of scientists has used a wireless brain-spinal interface to bypass spinal cord injuries (SCIs) in a pair of rhesus macaques, restoring intentional walking movement as early as six days after a spinal cord injury that paralyzed the animals’ legs. The researchers, who describe their work in the journal Nature, say this is the first time a neuroprosthetic device has been used to restore walking movement directly to the legs of nonhuman primates.
Scientists and neuroengineers from Ecole Polytechnique Federale Lausanne (EPFL), Switzerland; Brown University (Brown); Medtronic, Minneapolis, Minnesota; and Fraunhofer ICT-IMM, Mainz, Germany, collaborated on the study, and testing was conducted in collaboration with the University of Bordeaux, France; Motac Neuroscience, Manchester, England; and Lausanne University Hospital. The work, which builds upon neural technologies developed at Brown and partner institutions, could help in developing a similar system designed for humans who have had SCIs.
The brain-spinal interface is a pill-sized electrode array implanted in the brain to record signals from the motor cortex. The sensor technology was developed in part for investigational use in humans by the BrainGate collaboration, a research team that includes Brown, Case Western Reserve University, Massachusetts General Hospital, the Providence VA Medical Center, and Stanford University. The technology is being used in ongoing pilot clinical trials, and was used previously in a study led by Brown neuroengineer Leigh Hochberg, MD, PhD, in which people with tetraplegia were able to operate a robotic arm simply by thinking about the movement of their own hand.
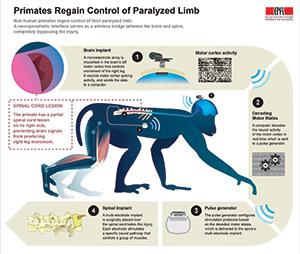
A visual diagram of how the system works, courtesy of EPFL.
A wireless neurosensor, developed in the neuroengineering lab of Brown professor Arto Nurmikko, PhD, sends the signals gathered by the brain chip wirelessly to a computer that decodes them and sends them back wirelessly to an electrical spinal stimulator implanted in the lumbar spine, below the area of injury. That electrical stimulation, delivered in patterns coordinated by the decoded brain, signals to the spinal nerves that control locomotion.
To calibrate the decoding of brain signals, the researchers implanted the brain sensor and wireless transmitter in healthy macaques. The signals relayed by the sensor could then be mapped onto the animals’ leg movements. They showed that the decoder could accurately predict the brain states associated with extension and flexion of leg muscles.
“The system we have developed uses signals recorded from the motor cortex of the brain to trigger coordinated electrical stimulation of nerves in the spine that are responsible for locomotion,” said David Borton, PhD, assistant professor of engineering at Brown and one of the study’s co-lead authors. “With the system turned on, the animals in our study had nearly normal locomotion. There is evidence to suggest that a brain-controlled spinal stimulation system may enhance rehabilitation after a spinal cord injury…. Doing this wirelessly enables us to map the neural activity in normal contexts and during natural behavior,” he added. “If we truly aim for neuroprosthetics that can someday be deployed to help human patients during activities of daily life, such untethered recording technologies will be critical.”
The researchers combined their understanding of how brain signals influence locomotion with spinal maps developed by the lab that Grégoire Courtine, PhD, oversees at EPFL. The spinal maps identified the neural hotspots in the spine responsible for locomotor control, enabling the team to identify the neural circuits that should be stimulated by the implant. The researchers then tested the system on two macaques with lesions that spanned half the spinal cord in their thoracic spines. Macaques with this type of injury generally regain functional control of the affected leg in about a month or so, the researchers said. The team tested their system in the weeks following the injury, when there was still no volitional control over the affected leg. The study showed that with the system turned on, the animals began spontaneously moving their legs while walking on a treadmill. Kinematic comparisons with healthy controls showed that the lesioned macaques, with the aid of brain-controlled stimulation, could produce nearly normal locomotor patterns. However, the researchers were unable to record how much pressure the animals applied to their affected legs.
Courtine, a professor at EPFL and the International Paraplegic Foundation (IPF) chair in spinal cord repair, who led the collaboration, has begun clinical trials in Switzerland to test the therapeutic effects of the spine part of the interface in people with SCIs.
Editor’s note: This story was adapted from materials provided by EPFL and Brown.




